Antioxidants are used in cosmetic and dermatological products to protect sensitive components from oxidation – both in the product and when applied to the skin. In addition, antioxidants are often multifunctional active ingredients, often vitamins, which can scavenge free radicals produced in the skin and influence physiological processes.
Preparations for the prevention of premature skin aging are a typical field of application of antioxidants, which are available in very many natural and synthetic variants. The synthetic compounds also include derivatives of natural antioxidants that integrate into the physiology of the skin. They have an advantage over non-physiological representatives such as butylated hydroxytoluene (BHT).
Use not always sensible
Antioxidants are nowadays propagated for almost every skin condition. However, their uncritical use is not always justified. In healing processes, for example, which are known to be radical, they can be counterproductive, especially if they are used in high doses. In sun protection products, for example, they make no sense at all, since they are broken down particularly quickly there and, on the other hand, cannot be used in high concentrations. This is because under these conditions, undesirable radical chain reactions can occur.
Protection and stability
For the shelf life of topical products, it is important that the antioxidants on the one hand protect other components such as essential fatty acids, but on the other hand also have the highest possible stability themselves – especially if they are to serve as active ingredients in the skin. To this end, there are a number of possibilities for the development of topical preparations.
Derivatives – chemical protection
The stabilisation of cosmetic ingredients through the aforementioned derivatisation occurs frequently. This is the chemical reaction of the reactive group of a substance with another substance, for example the hydroxyl group (HO) of tocopherol (vitamin E) with a physiological acid such as acetic acid.
In this case, an ester such as tocopherol acetate is formed from both components. In the case of tocopherol, the ester (tocopherol acetate) in the topical product itself no longer has an antioxidant effect.
The antioxidant property is restored on the skin by enzymatic cleavage of the ester into the parent components. Antioxidant alias reducing vitamins are similarly protected. Examples:
- Vitamin A (retinol): Retinol ester – INCI e.g. Retinyl Acetate, Retinyl Palmitate
- Vitamin C (ascorbic acid): Ascorbic acid ester – INCI e.g. Ascorbyl Palmitate, Ascorbyl Phosphate
- Vitamin E (Tocopherol): Tocopherol esters – INCI e.g. Tocopheryl Acetate, Tocopheryl Palmitate, Tocopheryl Linoleate
Derivatisation is also widespread in nature, mainly through the formation of glycosides, i.e., the coupling of antioxidants to saccharides. The natural substance arbutin, which is used in cosmetics for pigment disorders, consists of the strong antioxidant hydroquinone (banned in cosmetics), which is glycosidically bound to glucose (grape sugar). Incidentally, many reducing sugars protect themselves through intramolecular cyclisation. In this process, the original reducing aldehyde function is converted into a cyclic hemiacetal that only contains hydroxyl groups (Fig. 1).
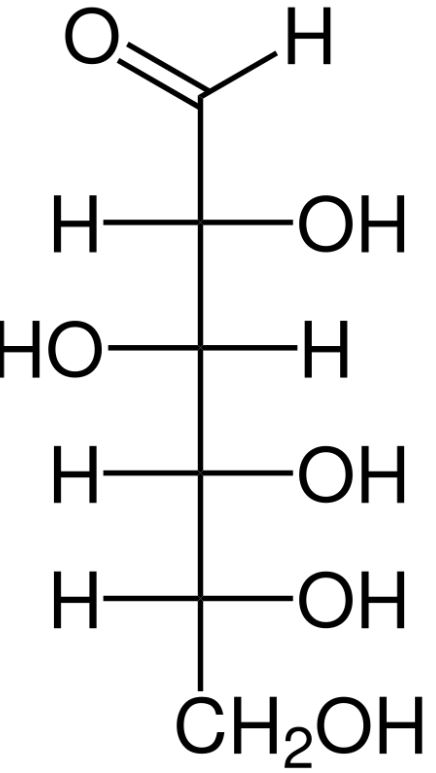 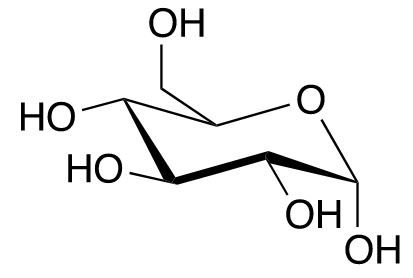
Glucose (aldehyde) → Glucose (hemiacetal)
Fig. 1: Intramolecular cyclisation of glucose
Encapsulation – physical protection
A certain protective effect results from the encapsulation of water-soluble antioxidants in liposomes or lipophilic antioxidants in nanodispersions.
In addition to protection, the formulations ensure penetration enhancement through the phospholipids contained in the enveloping single (monolayer) and double (bilayer) membranes. Most of these are phosphatidylcholine with a high proportion of bound linoleic acid.
In anhydrous products such as powders, antioxidants are often found in a protective matrix of polysaccharides – such as cellulose, starch or pectins and their derivatives.
Change of the redox potential
Another physical decrease in the sensitivity of antioxidants, or more precisely the change in their redox potential, can be achieved by pH changes. In the case of ascorbic acid, the antioxidant potential decreases when the pH is lowered, while it increases when the pH is raised. A vitamin C formulation with a low pH and low antioxidant potential will increase the pH of the skin a short time after application. This in turn increases the antioxidant potential.
Protection through additives
The formation of radicals – both in products and on the skin – is catalysed by heavy metal traces such as iron compounds in the presence of radiation. Since heavy metal traces are ubiquitous and their entry into topical products during removal and on the skin cannot be prevented, they naturally also cause the degradation of antioxidants.
Therefore, complexing agents are used as additives that are able to mask the heavy metals and remove them from circulation. For this purpose, one uses phosphoric acid, citric acid, tartaric acid salts or ethylenediaminetetraacetic acid, abbreviated EDTA.
While phosphates, citrates and tartrates are physiologically compatible, EDTA is not physiological, is difficult to degrade and thus pollutes water bodies.
(Poly-)saccharides like hyaluronic acid also exert a weak complexing effect on heavy metals. Since they themselves belong to the radical scavengers, they are suitable as a matrix for combinations with antioxidants – especially as protective films on the skin surface. This also applies to polyethylene glycols (PEG) with their characteristic building element (-CH2-CH2-O-)n. PEGs can chelate the heavy metals similar to crown ethers (cyclic ethers whose schematic structure in the sequence of ethylene oxy units (-CH2-CH2-O-) is reminiscent of a crown).
However, PEGs are not broken down in the skin and easily form peroxides on the skin surface if they are not themselves protected by antioxidants. In addition, PEGs easily dissolve nickel ions, which are still common in today's costume jewellery, and can be the cause of nickel allergies.
A good combination with antioxidants are amino acids, which in turn scavenge nitric oxide radicals (NO, NO2) or the resulting nitrite anions from the air, forming hydroxy acids. In addition to moisture binding, the skin's Natural Moisturising Factor (NMF), which consists largely of amino acids, also has this property. In some cases, antioxidants are protected by combinations of each other – for example, low concentrations of vitamin A (retinol) are protected by high concentrations of vitamin C or its derivatives. Sometimes oxidised forms are also used, such as retinal (aldehyde), which is formed by oxidation of retinol (alcohol). Retinal is the precursor of retinoic acid (vitamin A acid; INN: tretinoin), which is banned in cosmetics.
Moderate concentration
The reactions of antioxidants with radicals usually take place via radical intermediates. Tocopherol also forms a radical intermediately, but it is not very reactive and accordingly has a relatively long life. The situation is similar for other antioxidants. However, if high concentrations of antioxidants are used, radical chain reactions can be stimulated which by their very nature have a counterproductive effect. In particular, if external radiation also acts on the product, as was described at the beginning for sunscreens, a pro-oxidative state is quickly reached which turns the actual goal into its opposite and must be avoided at all costs. Here, too, the principle applies: less is more.
Packing
High-energy radiation from the sun and light contributes to the degradation of antioxidants. To shield products from radiation, containers are used that can reflect or absorb radiation. Violet glass or black glass are used for absorption.
Laminates made of plastic and aluminium foils are used for flexible tubes and dispensers. They are opaque and prevent the diffusion of substances – including gases such as oxygen and water vapour – from the outside to the inside and vice versa.
Since atmospheric oxygen, especially the photochemically produced, energy-rich singlet oxygen (1O2), reacts with oxidation-sensitive substances, some products are filled into ampoules under inert gas (carbon dioxide, argon), which in turn can often be made of amber glass. Glass snap-off ampoules are only suitable for single-use applications or must be used up within a short time.
Storage
Since the sensitivity of antioxidants depends on temperature, some manufacturers state on the packaging that the products should be stored in a cool place – e.g., at refrigerator temperature. For cosmetics, the rule of thumb in this regard is that the shelf life, based on room temperature, doubles when lowered by 10 degrees. However, it should be noted that the temperature must not reach ranges in which individual components, ultimately also water, crystallise out. Crystallisation is usually not reversible and, among other things, emulsions are destroyed and the physical shelf life suffers as a result.
Dr Hans Lautenschläger
|

