Hyaluronic acid1,2 alias hyaluronan is an endogenous sugar-like biopolymer consisting alternately of D-glucuronic acid and N-acetyl-D-glucosamine units (Figure 1). The name hyaluronic acid is derived from the Greek term for "glassy" and indicates its presence in the vitreous body of the eye. Hyaluronic acid is a ubiquitous lubricant in the joints and an important building block of the extracellular matrix and connective tissue of the skin. The density of hyaluronic acid in the skin decreases with age. This is counteracted by wrinkle injections of hyaluronic acid and its partially cross-linked derivatives in aesthetic medicine.
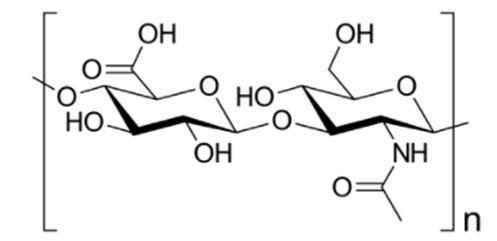
Fig. 1 Disaccharide repeating unit of hyaluronic acid: D-glucuronic acid (left) and N-acetyl-D-glucosamine (right).
In cosmetics, the sodium salt of hyaluronic acid (INCI: Sodium Hyaluronate) is usually used. Due to the extremely high water-binding capacity of hyaluronic acid3, a pleasant moisturising film develops on the skin. In the process, the hyaluronic acid bonds with the keratin through hydrogen bonding and creates a slight tension that smoothes out small wrinkles. The film also leads to a reduction in the depth of wrinkles.
Hyaluronic acid is produced biotechnologically with the help of bacterial cultures that produce polymer chains of several million Daltons (Da).
Experimental studies with fragmented hyaluronic acid show that at sizes of 50,000 and 130,000 Da ("low molecular") compared to "high molecular" hyaluronic acid (approx. 2,000,000 Da), there is an increase in skin hydration and elasticity and a decrease in wrinkle depth.4 It was concluded from this that low-molecular hyaluronic acid of this size can penetrate the skin barrier.5 Biophysically, however, the passage is not possible with an intact skin barrier.
The extent to which much smaller as well as larger fragments were present in the Gaussian size distribution of the material used was not investigated. It is also unclear whether the microbiome of the skin contributes to further degradation of the fragments. Ultimately, the smallest fragments are glucuronic acid and N-acetyl-D-glucosamine with 221 Da and possibly even glucosamine with 179 Da. These molecules can pass through the skin barrier and stimulate endogenous hyaluronic acid production.6,7 Suitable penetration enhancers for the passage of N-acetyl-D-glucosamine in skin care products are liposomes containing phosphatidylcholine and D-panthenol (provitamin B5).
Alternatives to attempts to transport hyaluronic acid or its fragments through the skin barrier are to stimulate the formation of endogenous hyaluronic acid by isoflavonoids or sapo(ge)nins, or to influence its degradation by inhibiting endogenous hyaluronidases, in addition to applying N-acetyl-D-glucosamine. Isoflavonoids useful for stimulation are found in soy and red clover extracts. Suitable saponins are found in butcher's broom extracts. The disadvantage of degradation-inhibiting substances such as heparin, pectins and alginic acids (brown algae) is their also high-molecular structure. The conditions are more favourable for liquorice root saponins such as glycyrrhizin and glycyrrhetinic acid (aglycone) as well as the aescin of horse chestnut, but as with all other substances, only in vitro evidence is available.
Applications and claims
Hyaluronic acid is suitable for the treatment of "dry eye", which manifests itself through redness and inflammation, especially when working at a computer screen, low room humidity and insufficient tear secretion. Hyaluronic acid solutions mixed with phosphatidylcholine liposomes are sprayed onto the closed eye. A similar application takes place with dry and possibly inflamed nasal mucous membranes by spraying hyaluronic acid together with D-panthenol into the nose, preferably in the evening before going to bed.
Like many polysaccharides, hyaluronic acid is a radical scavenger that acts on the skin surface. This results in a low protective effect against oxygen radicals resulting from UV radiation. The heavy metal traces involved in radical formation, such as iron, are complexed. Hyaluronic acid is easily removed when the skin is cleansed with water. It must be reapplied again.
Hyaluronic acid is offered in food supplements. Claims such as "for young skin" or "maintaining good skin hydration" have not been judged proven by the European Food Safety Authority (EFSA) even in the presence of an alleged Japanese study to the contrary. According to Regulation (EU) No. 1066/2013, these claims – if not expressly permitted ("reservation of permission") – are prohibited. In the advertising for corresponding capsules, therefore, the description of hyaluronic acid deficits that occur due to the ageing process of the skin is used. Incidentally, it is assumed that the amino sugar D-glucosamine has a life-prolonging effect.8
A review article on the chemistry and physiology of skin care has recently been published.9
Literatur
1 Lautenschläger H, Hyaluronsäure – ein legendärer Wirkstoff, Kosmetische Praxis 2008, 4, 16-18.
2 Lautenschläger H, Hyaluronsäure und Polysaccharide – für Hautfeuchte und gegen Falten, medical Beauty Forum 2018, 6, 15-18.
3 Hyaluronsäure und Haut, Trends in Clinical and Experimental Dermatology, Volume 3, Volume Editors: Wohlrab W, Neubert RRH, Wohlrab J, Shaker Verlag, Aachen 2004.
4 Pavicic T, et al., Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment, Journal of Drugs in Dermatology 2011, 10 (9), 990-1000.
5 Kaya G, Tran C, Sorg O et al., Hyaluronate fragments reverse skin atrophy by a CD44-dependent mechanism, PLoS Med 2006, 3, e493.
6 Uitterlinden EJ et al., Glucosamine increases hyaluronic acid production in human osteoarthritic synovium explants, BMC Musculoskelet Disord. 2008, 9, 120.
7 Sayo T, Sakai S and Inoue S, Synergistic effect of N-acetylglucosamine and retinoids on hyaluronan production in human keratinocytes, Skin Pharmacol Physiol. 2004, 17 (2), 77-83.
8 Bell, GA et al., Use of glucosamine and chondroitin in relation to mortality, Eur J Epidemiol 2012, 27, 593-603.
9 Lautenschläger H, Die Haut und ihre Pflege, Chemie in unserer Zeit 2021, 55 (5), 306-319; https://doi.org/10.1002/ciuz.202000057.
Dr Hans Lautenschläger |

