Betwixt and between: When it comes to the genera of chemical elements, it is about metals or non-metals. In between there are various metalloids with very individual features. This also applies to silicon, the second most common element of the earth crust.
The term Silicon Valley refers to the metalloid and is linked to the use of silicon (Si) since the chips of the IT industry are unimaginable without it. When it comes to popularity, elemental silicon only is outpaced by its oxide. Silicon dioxide (SiO2) is the base material of all sandy beaches and thus very popular with kids but also with adults. Sand is the alluvial debris that remains after the erosion of mountains and from a chemical point of view it is identically equal to quartz. Small amounts of it are hydrated in the water bodies in the form of dissolved silicic acid.
Silicic acid
Already dissolved silicic acid (H4SiO4) shows the propensity of silicon to interlink via oxygen bridges. Thus with increasing concentration di-, tri- and polysilicic acids and last but not least silica gel form with elimination of water. Silica gel is the amorphous equivalent to quartz with the following structural formula:
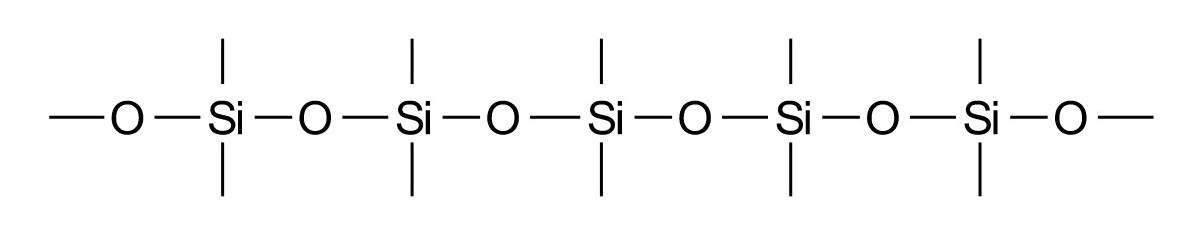
Due to its water-absorbing features silica gel is used as a dehydrating agent. Kieselgur has a similar structure. It is a highly porous SiO2-powder (INCI: Silica), forms from the erosion of diatomaceous earth and serves as an absorbent. The synthetic ultra-pure variant is aerosil, a typical thickening agent for paraffin-based oleogels.
Silicates
When individual Si-atoms in the Si-O-chain are replaced by aluminium (Al), alumosilicates form as for instance clay, kaolin (alias white clay or china clay), argil (or potter’s clay), bentonite (clay-like mineral with large surface) and mountain-forming (or orogenetic) mica and feldspars that are accompanied by additional elements such as calcium, barium, sodium and potassium. Zeolites also belong into this category. Due to their pore-like tubes into which other substances can be absorbed and released, they serve as absorbents, ion exchangers and catalysts.
In the focus of interest also are the absorbing features of healing earths consisting of clay or (clay-bearing) loam. Healing earths are used as topical, oral and physiotherapeutic remedies.
Formally speaking, silicates are the salts of silicic acid. Talc occurs in compact form as soapstone (alias lard stone), is on the market in triturated form as talcum powder and consists of magnesium silicates. Asbestos modifications also belong to this category. Since powdery silicates cannot be degraded by humans, not only the fibrous asbestos particles but also the respirable fractions of other silicates can cause granulomas. This is the reason why powders often are promoted as talcum-free.
Because of the absorbing properties and -capacity for cosmetic active agents silicates are predestined for the use as inorganic components of masks and packs. The pigments used for make-up products are based on silicic acid or mica and covered with coloured oxides. They achieve particular optical effects up to optical wrinkle reductions.
Silicon in lively nature
All plant- and animal-based food contain small amounts of silicic acid respectively silicates. Grasses, horsetail and bamboo use silicic acid to harden their stems. Brown algae (alias diatoms) also build up SiO2 structures. The relevance of silicic acid for the human organism that only contains some grams of it, still today is of speculative nature. But one never knows, one of these days researchers may find the bio-chip in our brains.......
Organic silicon compounds
Compounds of silicon-, carbon- and hydrogen atoms among each other fall into the category of organic chemistry. The most diverse group among them is the group of silicones. It should however be mentioned that most of the silicone compounds in fact are siloxanes in which the silicon atoms are linked via oxygen atoms, similar to the inorganic SiO2. The most basic representative of these compounds is Hexamethyldisiloxane (INCI) with the structural formula:
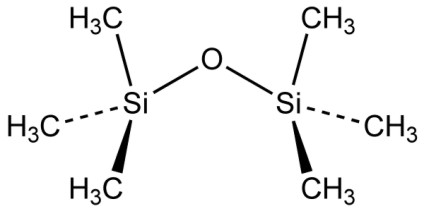
It has a boiling point of 101 °C and is used as a solvent as for instance in antiperspirants from which it escapes like water after its application however without the cooling features.
The term Dimethicone (INCI) used for linear chain-like (poly-)siloxanes comprises representatives from liquid to waxy texture, analogous to the paraffin chains that have similar lipophilic properties. They still are used in water-repellent skin protection preparations provided that annoying fingerprint marks are no disqualifying criterion. Further fields of application are skin care preparations with velvet-like haptic and hair care products; they are also used as spreading- and plasticizing components. The indicated chain lengths of higher molecular dimethicones usually are average values, in other words, they are compounds.
The cyclic variants of dimethicones with 6-10 links are called Cyclomethicone (INCI). Their fields of application are similar to dimethicones, among others, they also were ingredients of lipsticks, however due to their teratogenic effects they have recently been withdrawn from circulation.
Other siloxane variants are e.g.:
- Alkyl Dimethicone, in which the methyl groups are partly substituted by longer hydrocarbon residues (alkyl). The alkyl residues are named first in the INCI list. They are called Alkyl Methicone when one of the two methyl groups on the silicon atom is completely replaced by alkyl residues.
- Phenyl Methicone (INCI): besides the methyl group they also contain a phenyl group. They improve the silkiness and shine of the hair.
- Additional hydrophilic properties can either be obtained with hydroxylated alkyl groups such as Hydroxypropyl Dimethicone (INCI) or with free Si-OH on the ends of the chains (Dimethiconole [INCI]). Dimethicone Copolyole stabilise the foams in cleansing preparations. They have copolymerised polyethylene glycol (PEG) chains and also can serve as emulsifiers.
- In combination with quaternary ammonium groups, cationic polysiloxanes such as Quaternium-80 (INCI) are formed. They are used as hair conditioners.
- The combination of siloxanes with silicic acid results in Silica Dimethyl Silylate (INCI). They serve as lipophilic consistency agents.
Although they are not physiological substances and higher concentrations in skin care preparations impair the endogenous skin regeneration, long-chained siloxanes are very well tolerated by the skin. A disadvantage however is the non-degradability or extremely slow degradability of siloxanes. The abrasive effect of the forming SiO2 during refuse gas incineration damages the sewage disposal facilities, which is why the contained siloxanes have to be elaborately filtered out with activated carbon treatment. The siloxanes used for domestic sealing and weather-stripping purposes interlink and solidify with elimination of either volatile acetic acid or esters with very characteristic odour.
Dr Hans Lautenschläger | 
