The wax-like white and both the solid red and black modifications do not necessarily imply that phosphorus (P) is closely related to the inert and gaseous nitrogen. The element does not occur in its free form in natural surroundings. White phosphorus easily ignites in the open air and develops intense heat when it burns. The oxide forming in this process forms phosphoric acid in presence of water. Red phosphorus is one of the components of the striking surface of a matchbox.
Bones and teeth
Worth mentioning among the phosphates, i.e. the salts of phosphoric acid are the apatites gained by mining. Hydroxylapatite is a calcium phosphate and the base substance of bones and teeth. It forms from the water-soluble phosphates that we ingest with our daily nutrition. At the bottom of the food chain are microorganisms and plants with their roots absorbing the mineral phosphates in the soil.
Energy storage
A specific feature of phosphoric acid (H3PO4) consists in forming diphosphoric acid (H4P2O7) and triphosphoric acid (H5P3O10) with release of water and energy absorption. This principle helps organisms to store and transport energy which they release later on with the intake of water and the formation of monomeric phosphoric acid. The energy stores supply the energy for activating enzymatic processes or in other words, for all kinds of metabolic activities. The optimized process in this context is the linkage of phosphoric acids to the sugar residues (ribose) of the nucleosides adenosine (A), guanosine (G), uridine (U) and cytidine (C).
The main energy supplier is the nucleotide adenosine triphosphate (ATP) which is formed in the energy-producing mitochondria of cells. Coenzyme Q10 that already is known from cosmetic applications participates in this process. The energy again is released through gradual enzymatic cleavage of phosphoric acid molecules. In this way adenosine phosphate (ADP) and adenosine monophosphate (AMP) form from ATP.
The other nucleotides, as for instance guanosine triphosphate (GTP), uridine triphosphate (UTP) and cytidine triphosphate (CTP) show a similar behaviour. Phosphoric acid can also cyclically link up with the ribose of the nucleotide; cyclic adenosine monophosphate (cAMP) is a second messenger that passes on the arriving signals regarding the activation of cell reactions.
Nucleic acid
Nucleotides are the components of ribonucleic acids (RNA) in which phosphoric acid is the linking chain between the sugar residues.
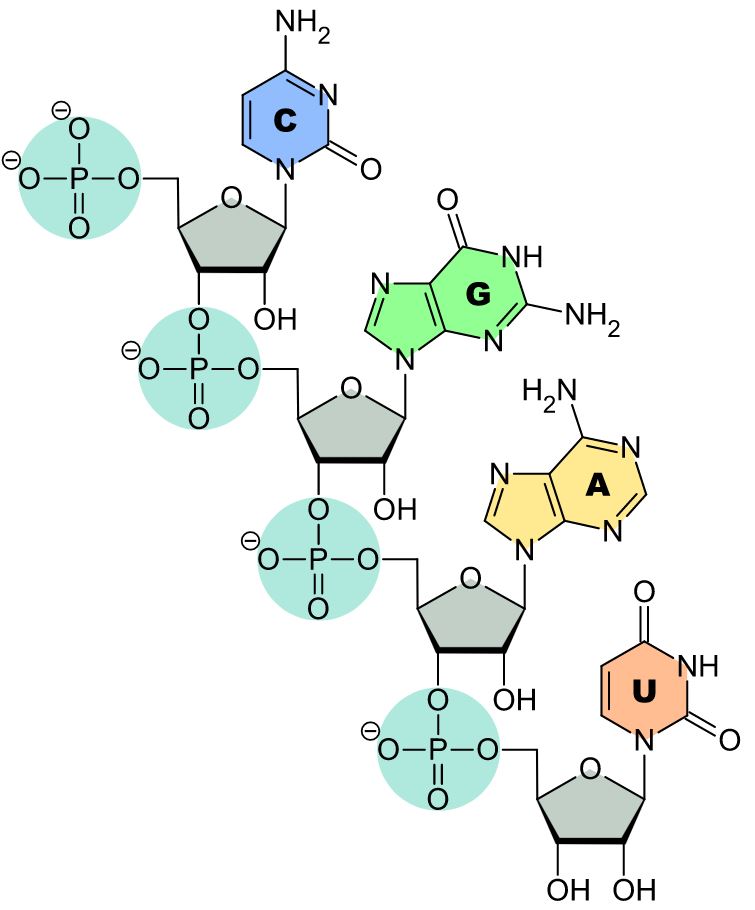
RNA-prinziple
(Wikipedia Commons, https://de.wikipedia.org/wiki/Ribonukleins%C3%A4ure)
In the nicotinamide-adenine-dinucleotide (NAD) that acts as an oxidation- but also as a reduction agent within cells, diphosphoric acid links up both the ribose residues. A similar behaviour shows nicotinamide-adenine-dinucleotide-phosphate (NADP) which has an additional phosphate residue linked up to the ribose group.
pH stabilisation
Dihydrogen- and hydrogen phosphates result from the partial neutralisation of phosphoric acid. In the form of sodium- or potassium salts they serve for the pH stabilisation of cosmetic products. Such systems are called buffers. They intercept ("buffer") the acids that can form during the storage of cosmetic products and lead to the breakdown of emulsions. In addition to it, the phosphates inactivate contaminations of heavy metal ions such as iron that catalyse oxidations and radical formations.
Other than that, phosphates occur in makeup preparations in the form of purple- (CI 77 742; manganese ammonium diphosphate) and red pigment (CI 77 745; manganese phosphate).
Phospholipids
Phosphoric acid esterified with the trivalent alcohol glycerine and organic bases such as choline (trimethylaminoethanol) has particular relevance. Such compounds are called phospholipids and contain long-chained fatty acids at the glycerine interface; they practically participate in all the membrane structures of cells. They occur as matrix elements, where chemical reactions take place, and also are found in the cell membranes where the controlled physical exchange of substances between inside and outside cells happens.
In dermatological cosmetics they occur in lamellar creams or in other words creams with a structure similar to the skin barrier and are a basic requirement for the transport functions of liposomes and nanodispersions. Most important representatives are phosphatidylcholine (PC) made from soya with integrated essential ω-6- and ω-3-fatty acids, and the hydrogenated and also physiological version with saturated fatty acids such as stearic- and palmitic acid. PC is successfully used in the concomitant skin care in the case of acne and also in the treatment of the dry eye syndrome, among other applications. The related intracellular sphingomyelins in which glycerine is substituted by sphingosine also are of physiological significance. They are the basic material for the formation of the barrier-stabilising ceramides during the apoptosis of skin cells.
Anionic emulsifiers
Phosphoric acid esters also occur among synthetic emulsifiers. Corresponding typical, long-chained alcohol components for instance are hexadecanol (C16) and octadecanol (C18). Acidic esters such as hexadecyl phosphate alias cetyl phosphate belong to the group of anionic emulsifiers which are activated through neutralisation with sodium- or potassium hydroxide.
Phosphonic acids
Besides the phosphoric acid esters with carbon-oxygen-phosphorus-bonds (C-O-P) there are also direct carbon-phosphorus-bonds (C-P). They are called phosphonic acids and can be components of herbal and animal organisms. A synthetic variant is etidronic acid alias 1-hydroxyethane-(1,1-diphosphonic acid) which is used in hair care products and in soaps for water softening purposes and also serves for the interception of heavy metal traces. Diphosphonates are used for the treatment of osteoporosis patients. The herbicide glyphosate also is a phosphonic acid with the phosphonic acid bonded to the amino acid glycine [N-(Phosphonomethyl)glycine].
Oral care
Salts of the fluorophosphoric acid are used for the oral care and particularly in tooth pastes for the prevention of caries. Just to mention some examples: ammonium-, sodium-, potassium- and calcium-mono fluorophosphate. The fluorine content in the preparation should not exceed 0.15%. Further restrictions apply to children under 6 years.
Combat agents1
Many lethal combat agents are phosphoric substances with the toxins absorbed via skin, respiratory system or with oral intake. The neurotoxins already are effective in the µg-range – just as the representatives of the Nowichok group that only recently were in the news. A typical feature of these substances are nitrogen-phosphorus- (N-P) and fluorine-phosphorus-bonds (F-P) or sulphur-phosphorus-bonds (S-P) as used during World War II for the VX-combat agent.
Structurally related to nitrogen mustard, a combat agent during World War I is the pharmaceutical drug cyclophosphamide which is an alkylating cytostatic.
1) blue: Not included in the publication
Dr. Hans Lautenschläger | 
