|
Aldehydes and ketones are oxygen-containing hydrocarbons and can be found in abundance either in natural surroundings but also in combination with chemical processes. They are formed with the oxidation of alcohol for instance. The low molecular molecules are used in cosmetic products because of their flowery notes and solvent characteristics. Aldehydes and ketones also are known for their chemical reactivity which is the reason for a strong antimicrobial activity.
Formaldehyde is not equipped with an agreeable fragrance though, as a matter of fact even in small concentrations it has quite a disagreeable pungent and acrid smell that irritates eyes and respiratory tract. Due to the high reactivity of formaldehyde with nitrogen-containing substances like proteins respectively amino acids, it is used as a disinfecting agent and preservative.
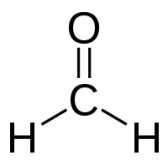
formaldehyde
Since formaldehyde has meanwhile been classified as a carcinogenic substance, its use in cosmetic products is subject to strong restrictions (KVO - German Cosmetic Decree): "Every finished product containing formaldehyde and those that release formaldehyde are subject to be labeled "containing formaldehyde" as far as the concentration of formaldehyde in the finished product exceeds 0.05 %." Nail hardeners may contain concentrations of about 5 % though. Once applied on the nails it cross-links with the protein structures of the keratin in the nails similar to cross-linkage processes in plastics manufacturing.
Formaldehyde use declining
Formaldehyde is less and less often used as a preservative in cosmetic products. It has to be mentioned though that quite a few formaldehyde releasers as e.g. imidazolidinyl urea are still being added. In case that this substance reacts within the skin, the complete formaldehyde content will be released. That is why formaldehyde releasers have the same allergenic potential as formaldehyde itself. Formaldehyde releasers are completely scentless though.
In the human body, acetaldehyde is formed during the oxidation process of ethanol. Traces may be contained in essential oils; however it is not used in cosmetic products. Its tetramer (metaldehyde) even is explicitly banned from cosmetic products.
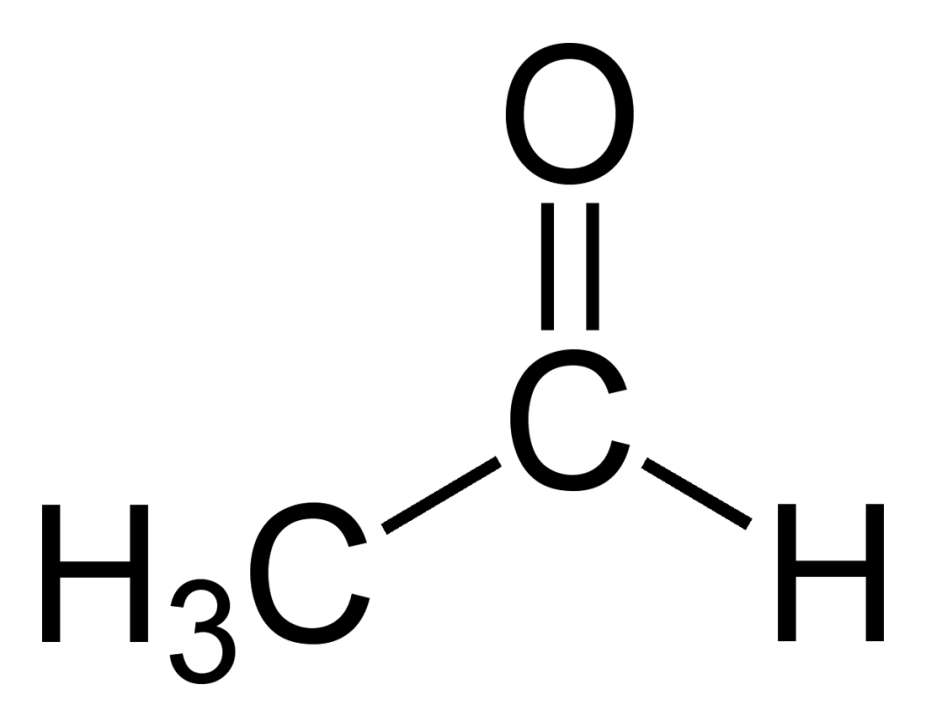
acetaldehyde
Acetone is a major solvent agent and used as nail enamel remover. In combination with the disinfecting, hair bleaching or universal bleaching agent hydrogen peroxide, highly reactive acetone peroxide (APEX) may form. That is why you should be careful with your waste material!
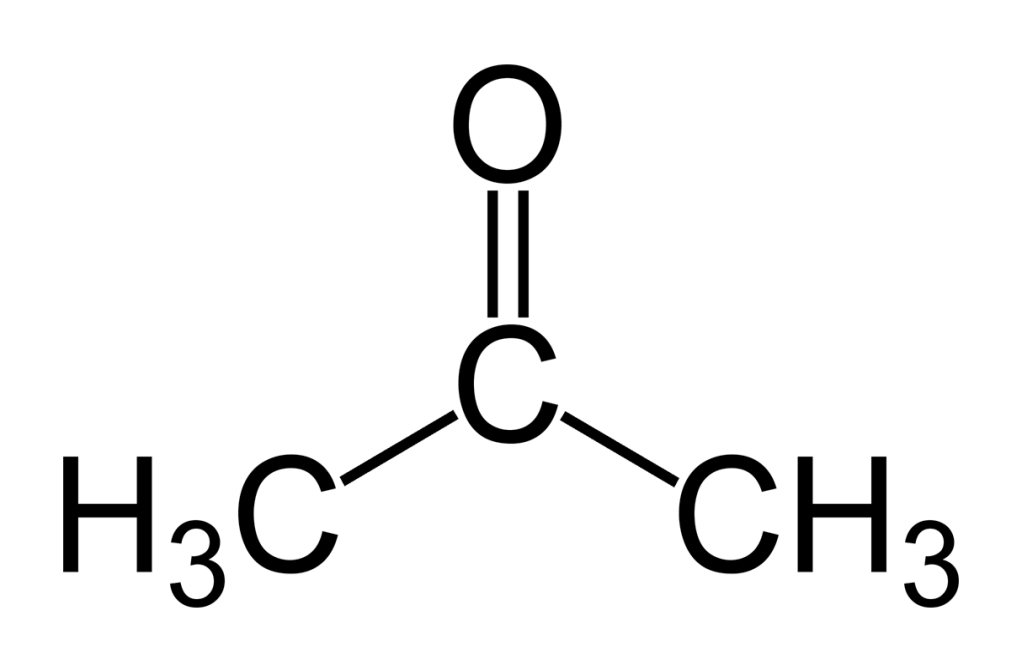
acetone
Glutaraldehyde belongs to the dialdehydes: both ends of a chain of 5 carbon atoms have a carbonyl function (C=O) attached. It is used for disinfecting and preservative purposes. Glutaraldehyde is banned in aerosols and the maximal concentration allowed is 0.1 %. It is subject to the warning label "contains glutaraldehyde".
Quite aromatic
Benzaldehyde is the most basic aromatic aldehyde. It smells like bitter almonds and occurs in essential oils. It should be mentioned though that the term "aromatic" does not refer to the smell but to the chemical structure of the "aromatic" benzene ring.
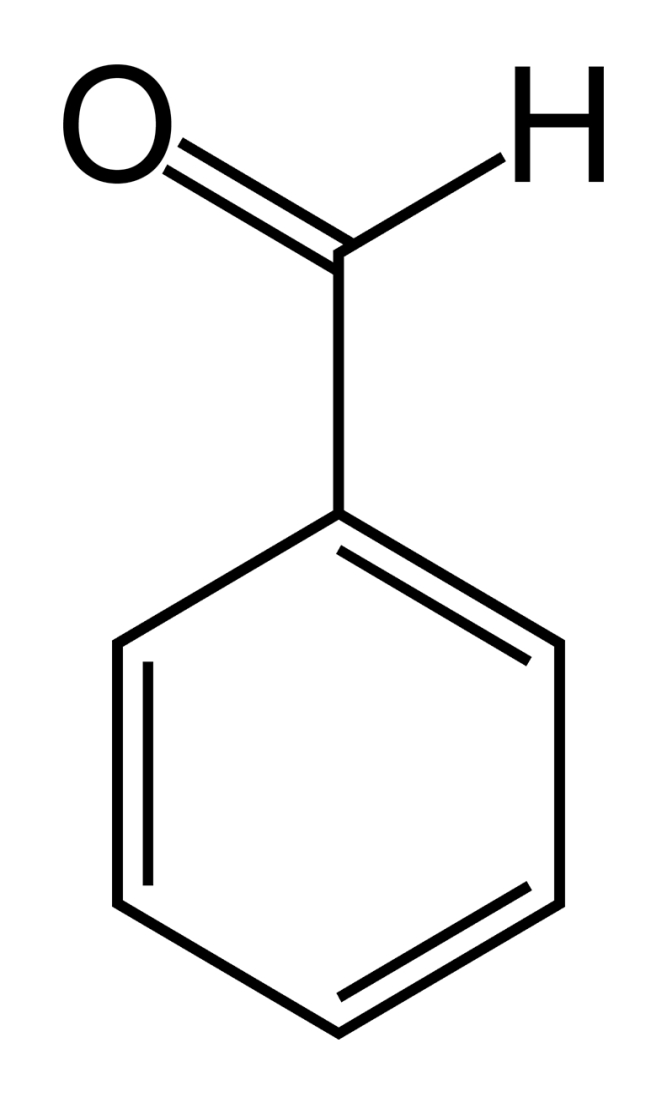
benzaldehyde
The substance is a rather versatile flavoring agent. Diluted it has a wild cherry-like scent.
In bitter almonds, apricot stones but also in apple cores benzaldehyde is chemically bonded to amygdaline and after the oral intake it is enzymatically hydrolyzed into glucose, benzaldehyde and prussic acid which also has a bitter almond scent.
Due to its vanilla and almond-like scent piperonal (heliotropin or 3,4-methylendioxybenzaldehyde) has traditionally been used to perfume simple soap bars. Piperonal has preservative properties and is sometimes used in combination with the lilac-like smelling hydrocinnamic alcohol ((3-phenylpropanol) as a preservative in skin care products. The aldehyde also is a base substance for hallucinogenic drugs (amphetamines, ecstasy) which is the reason why the trade in this substance is closely supervised also in the field of cosmetics.
Further natural aromatic aldehydes with characteristic scents are for instance anisealdehyde (4-methoxybenzaldehyde) as well as vanilline (4-hydroxy-3-methoxybenzaldehyde).
Caution: allergenic
Cinnamic aldehyde (Cinnamal) is a cinnamon and balsamic-smelling component of natural essential oils with highly allergenic potential. This is the reason why it has to be labeled separately in the INCI list if concentrations of more than 0.001% are contained in products that remain on the skin respectively more than 0.01% in rinse-off products. The same applies for 2-benzylideneheptanal (INCI: Amyl Cinnamal) and 2-benzylideneoctanal (INCI: Hexylcinnamaldehyde) with their flowery jasmine-like notes. They are structurally related to Cinnamal.
Besides a number of other ketones, muscone, a 15-membered cyclic ketone is the main component of natural musk. Today, muscone is exclusively gained from synthetic sources. The INCI term "Musk Ketone" refers to a synthetic ketone (4'-tert-butyl-2',6'-dimethyl-3',5'-dinitroacetophenone) which belongs to the controversially discussed nitro musk compounds. The German Cosmetic Decree (KVO) states that only up to 1.4 % may be contained in perfume, 0.56 % in Eau de Cologne and 0.042 % in other products.
Chinones are 6-membered cyclic unsaturated diketones as contained in coenzyme Q10 (Ubichinone) and in vitamin K.
The autoxidation of unsaturated acids and their triglycerides releases degradation products from which already minuscule concentrations can be noticed by the human olfactory sense. This rancid smell is almost exclusively ascribed to the secondary aldehydes and ketones forming in this process. The saturated aldehydes pentanal, hexanal and heptanal and specifically strong-smelling unsaturated aldehydes like 2-nonenal as well as the ketone 1-octene-3-on are formed from linoleic acid.
Essential oils
An exceptionally multifaceted group of aromatic substances are terpenes, the main components of essential oils. Among them also are numerous ketones which can generally be recognized by the suffix "-one" just as aldehydes with the suffix "-al". They are contained in fragrances and spices and quite often also have physiological effects:
2-Bornanone (camphor - resin of the camphor tree) has a eucalyptus-like scent and supports the micro circulation. It also has cooling, antiseptic effects.
(+)-Carvone (caraway oil, dill oil): releases a caraway-like scent
(-)-Carvone (crisped mint oil): The mirror image of (+)-carvone has a mint-like scent and also a highly allergenic potential
Carvenone (dill oil)
Fenchone (fennel oil, caraway oil)
ß-Ionone with a violet-like scent occurs in various plants
Junionone (juniper berry oil)
Menthone (geranium oil, peppermint oil) has a mint-like scent
Pinocarvone (eucalyptus oil) is the sexual hormone of the pine looper
Piperitone (eucalyptus oil)
Pulegone (pennyroyal oil): peppermint-like scent, irritates the skin
Thujone (thuja oil, sage oil): menthol-like scent
Verbenone (rosemary oil): mint-like scent belongs to the pheromones of the bark-beetle
Citral: is a mixture of citral A (geranial) and B (neral). Both have a lemon-like scent and are contained in lemon and lemongrass oil. According to the German Cosmetic Decree (KVO) they are subject to declaration.
Citronellal (lemon oil, lime oil)
Safranal (saffron oil): is the main component of saffron.
Hydroxycitronellal has a sweet, flowery scent. It is gained from synthetic substances and subject to declaration according to the German Cosmetic Decree (KVO).
Chemical change possible
In contrast to ketones, aldehydes can easily be oxidized into acids; that means they also have reducing characteristics. Formaldehyde changes to formic acid and acetaldehyde to acetic acid. With unprotected open storage especially the aromatic aldehydes may chemically change into the respective substituted benzoic acids. Accordingly, essential oils and perfumes that are rich in aldehydes may change their scent with extended storage.
The conversion products of 2 alcohol molecules with an aldehyde or ketone molecule have a particularly agreeable scent. Acetals and ketals are formed in this process while the water escapes. The substances also occur in natural surroundings and may easily be synthetically manufactured. They are frequent components of perfume compositions.
In cases where the remaining alcohol group of an aldose (carbonyl group at the end of a monosaccharide) or of a ketose reacts with an alcoholic HO-group, specific acetals or ketals will form which belong to the group of glycosides. This substance class is widely spread in natural surroundings (as e.g. in saccharose or lactose). After contact with acids, acetals, ketals and glycosides will decompose into their basic components. This is why they are also called disguised aldehydes and ketones. And by the way: vitamin C also is a disguised ketone.
Dr. Hans Lautenschläger | 
