Nanoparticles aren't as new as it may seem. They even have a longer history than we would think of. Since human beings have inhabited the earth they have been exposed to small particles. Particularly aerosols i.e. particles floating in the air, have surrounded them since the year one. Examples are smoke and soot developing with camp or chimney fires, dust from the Sahara which has traveled over hundreds of kilometers, loess that fertilizes the fields with its minerals and also volcanic ashes which has soared into high air layers. Every friction of natural or synthetic solid bodies generates tiny visible bodies but also miniature particles that cannot be detected with the human eye. Among aerosols and dusts nanoparticles are omnipresent. Optical features Nanoparticles are invisible since their diameter is smaller than the wavelength of the visible light which is about 400 to 800 nm with the unit "nm" as an abbreviation for "nanometers". Just to recall for comparison:
1 meter = 1 m
1 millimeter = 1 mm = 0,001 m = 10-3 m
1 micrometer = 1 µm = 0,000001 m = 10-6 m (diameter of human hair: 60-100 µm)
1 nanometer = 1 nm = 0,000000001 m = 10-9 m
1 Ångström = 0,1 nm = 0,0000000001 m = 10-10 m (average radius of atoms) Titanium dioxide nanodispersion which is used in sun screens is almost colorless as its particle size is below 400 nm. That is why sunblockers with a high concentration of titanium dioxide are no longer as obtrusively whitish as they used to be.
So called colloid-disperse systems consist of liquids that contain particles between 1 and 500 nm. They excel by their specific optical features. If their particle size attains the visible area of the light the particles can become opalescent which means that they take on a milky to colored glimmer. In apparently clear dispersions like soap solutions frequently the Tyndall effect can be observed. In this case we look sideways at the scattered light of a light beam, generated by the particles. Real solutions will not scatter visible light. Visible sun rays from a hole in the clouds or at a clearing in the woods are based on the same optical effect. The intensity of dispersion is stronger with smaller wavelengths; that is why nanoparticular colloidal dispersions frequently seem bluish. Sometimes also the color of a substance changes: colloidal gold dispersions with a particle size below 100 nm are dark red for instance. Multifarious applications There is a wide range of nanoparticles in the cosmetic and pharmaceutical field:
Solid nanoparticles
Metal oxides: titanium dioxide nanoparticles are used as a mineral sun protection. In cancer therapy ferrous oxide nanoparticles are injected and then selectively transported into the tumor tissue. There they are heated with alternating magnetic fields and thus damage the tumor tissue. Nano or microcapsules: consist of polymerisates, e.g. polypeptides with encapsulated pharmaceutical agents which then are slowly released (retard applications). In this case the small particle size generates a specifically large surface area which allows a controlled release of the active agents out of the polymer matrix. Hydrocarbons and waxes ("Lipopearls®"): together with cosmetic active agents these organic additives are melted at high temperatures in aqueous dispersions, adjusted to the required particle size in a homogenization process and then cooled down. In this step solid nanoparticles will form which are also called SLN (solid lipid nanoparticles). They combine on the skin into a surface film from which the active agents then are released - similar to an occlusive system.
"Fluid"or "liquid" nanoparticles
Membrane containing nanoparticles: they are oil bodies with lipid soluble active agents that are surrounded by a phosphatidylcholine (PC) membrane. With reference to liposomes they are sometimes also called nanosomes (see below).
Chylomicrons are natural carrier systems for lipids in the lymphatic system of the body. Besides PC the external membranes also contain transport proteins. Ceramides, phytosterols and fatty acids: in the nineties this type of compounds in membranes have become known as Nanoparts®. Liposomes are the bilayer variant of nanoparticles. Their structure is derived from natural cells. In contrast to nanoparticles in the narrower sense they have an aqueous interior and are predestined to encapsulate water soluble cosmetic and pharmaceutical active agents. Niosomes are the synthetic relatives of liposomes which consist of natural PC.
Variable particle size Besides the specific variety called liposomes which will not be considered in this context, cosmetics mostly uses solid nanoparticles in form of titanium dioxide and the PC containing fluid nanoparticles. Another however rarely used term for fluid nanoparticles is nanoemulsion. It has to be stated that phosphatidylcholine (PC) has very few in common with emulsifiers respectively emulsions though. As already mentioned above, PC as a natural substance of the body is responsible for the transport of lipids within the lymphatic system and the blood stream. PC is completely metabolized and provides the skin with two essential substances: linoleic acid and choline. The particle size of nanoparticles in aqueous dispersions is not consistent. As a rule, the average size is indicated which is calculated in a distribution curve with characteristic curve shape as shown in the following graphics: 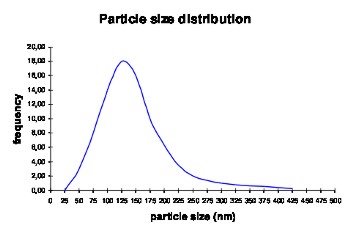 While the minimum level of the particle size sets in rather abruptly, a clear cut trail is noticeable in direction to the larger particles. In cases where the trail attains the visible area of the light or in other words where a small section of the particles is larger than 400 nm, the aqueous dispersions appear opalescent to milky, depending on the concentration of the larger particles. Average value and particle distribution both depend on the composition of the oily interior. There is a dynamic balance in fluid nanoparticles which means that in cases where the mixture is forced into a smaller particle size during the manufacturing process, it slowly converges back to the characteristic balance of the mixture when stored for a longer period.
In the majority of cases the particle size of nanoparticles and liposomes is measured with the "photon correlation spectroscopy (PCS)". It is used for particles which move in accordance with the Brownian motion when suspended in liquids. This applies for particles with a diameter of 2-3 µm and smaller. The intensity of the movement is inversely proportional to the particle size or in other words, the smaller the particles the faster they move.
The intensity of the movement can be measured by analyzing the time dependence of the intensity fluctuations of the laser light diffused through the particles.
The average particle size of commercial cosmetic liposomes is between 25 to 200 nm with a standard deviation between 5 and 30 nm. The average particle size of fluid nanoparticles is between 70 and 200 nm. Stable nanoparticle dispersions must not sediment (form deposits). The physical stability is measured with a centrifuge (between 5,000 and 10,000 rotations per minute [rpm]). In contrast to regular emulsions no structures can be detected with the microscope. Structures only become visible with the help of an electron microscope. The diameter of the PC shell of fluid nanoparticles ranges between 2-3 nm. The physiological side Usually cosmetic nanoparticles are supplied as serum or active agent concentrates. These are aqueous nanoparticle dispersions containing additives to control the consistency, as e.g. xanthan and other compatible water soluble substances.
In contrast, the nanoparticles of fine and ultrafine particulate matter are freely available. Once blown up in the air they can be a safety hazard due to the fact that they may infiltrate into the alveoles of the lungs when breathing and then trigger the well-known effects of non degradable solid matters like asbestos, glass fiber, carbon dust and diesel exhaust particles. Such nanoparticles do not occur in the daily use of cosmetics. Even the pigments of cosmetic powders contain substantially larger particles (> 5 µm) and will be retained at the latest along the bronchial tubes and then eliminated, in case that blown up powder is breathed in ("coarse dust"). Also titanium dioxide which is used in powders as a whitening pigment is more coarse grained than the transparent alternative applied as UV filters in sun protection products.
Down to the present day there are no empirical findings that cosmetic nanoparticles like titanium dioxide or zinc oxide embedded in the matrix of sun protection creams may penetrate into the skin. This also applies for the diseased skin, as e.g. psoriasis. Non mineral solid nanoparticles based on solid hydrocarbons, waxes, including the additives used in the process are also blocked off by the horny layer. As already mentioned above they aggregate to form superficial films and then release their active agents into the skin. Hence they will act like emulsifier free creams with a high percentage of mineral oil components and waxes which however cannot be physiologically exploited.
Quite different are fluid nanoparticles and their hydrophilic relatives, the liposomes. They penetrate into the barrier layers of the horny layer where they dissolve immediately due to their specific composition. During this process a fluidization of the skin barrier layers takes place and the encapsulated active agents can pass through the skin barrier. The following figure gives a basic idea of the process in case of liposomes: Figure:
liposome approaches
the skin barrier layers
Figure:
liposome fuses with
the skin barrier layer
Figure:
liposome has dissolved and
released the active agents
PC in the shell of fluid nanoparticles occurs as monolayer and not as bilayer as in liposomes. It acts however in the same way. After its primary fusion with the barrier layers it penetrates into the deeper skin layers or is enzymatically and hydrolytically split into the physiological components like fatty acids, glycerin, phosphoric acid and choline. This is the reason why the barrier function of the skin is restored after a few hours.
A specific advantage of fluid nanoparticles consists in the fact that besides lipophilic active agents particularly fattening natural oils can be forced into a sensorially agreeable aqueous dispersion without adding synthetic and barrier disturbing emulsifiers and which easily penetrates into the skin.
Much ado about nothing? With all the commotion about small particles it should always be kept in mind that the skin continuously is exposed to substances of the size of molecules. Depending on their size and polarity they more or less pass through the skin barrier or are completely retained. While cosmetic nanoparticles frequently occur in sizes of about 100 nm, small molecules occur in sizes less than 1nm. Atoms and ions with about 0.1 nm are even smaller. The ionically built and in water dissolved table salt is about in the same order. In this context however nobody would argue that table salt is dangerous. Hence the considerations that apply for molecules should also apply for nanoparticles.
Dr. Hans Lautenschläger | 
