Steroids are a specific substance group which is characterized by a specific molecular structure. A typical representative of this group is cholesterol:
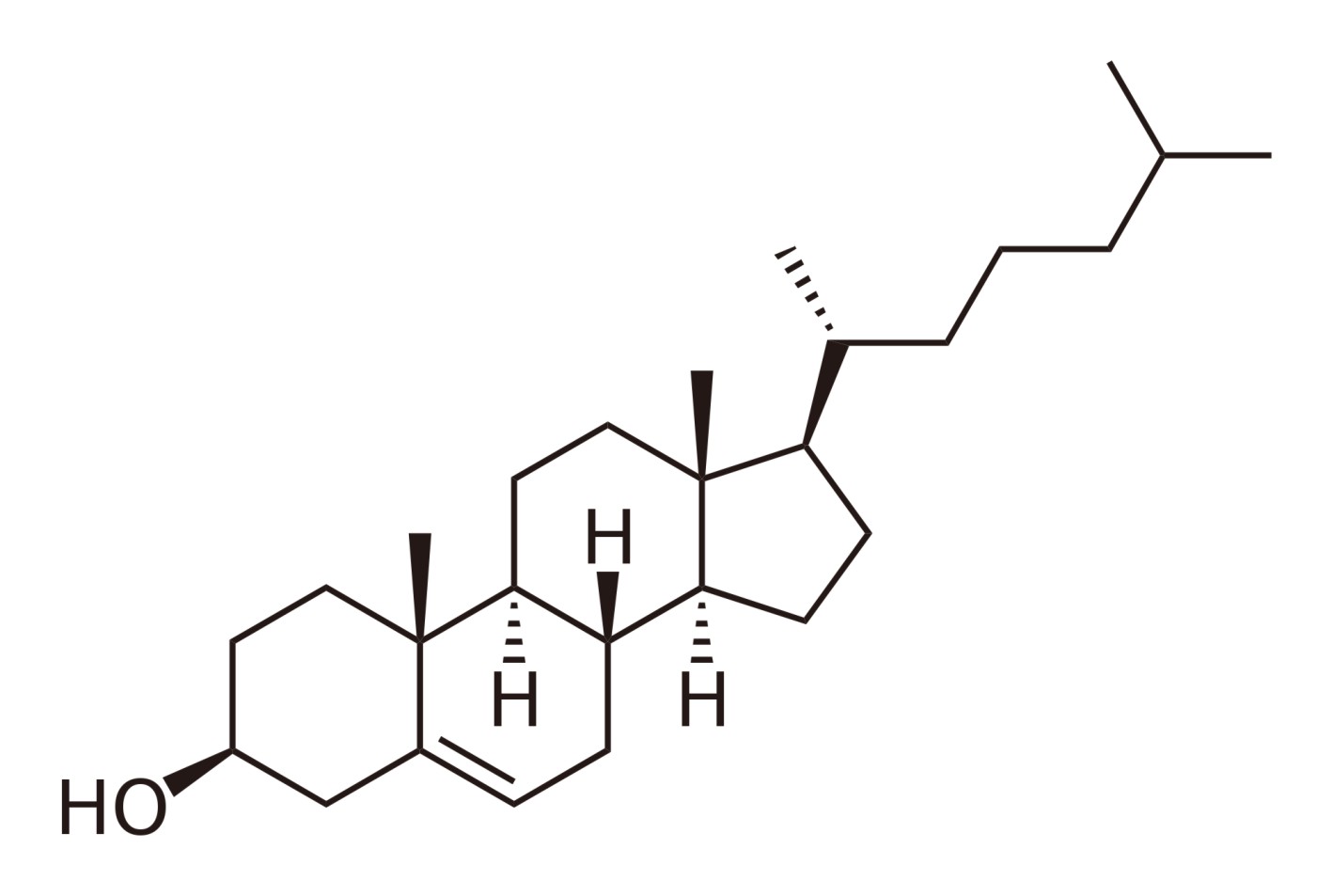
The word steroid is derived from "sterane" which is the technical term for the 4 linked carbon rings that form the basis. The individual representatives have different side chains and functional groups.
Lanolin
Cholesterol contains a hydroxy group that also provides slightly hydrophilic features to a substance which otherwise is structured like a hydrocarbon and hence a liposoluble substance. This specific feature increases the water retaining capacity of wool fat (adeps lanae) in which it is contained. Lanolin is the term for a mixture of wool fat (65 grams), paraffin oil (15 grams) and water (20 grams) and a frequent component of W/O emulsions in pharmaceutical skin ointments. In the cosmetic field, wool fat and lanolin are synonyms. Cholesterol has excellent skin protecting effects and is a component of the natural skin barrier.
Contaminations of wool fat resulting from the use of pesticides for instance can nowadays be excluded due to the high quality standards of the product. Hence, individual allergic reactions are extremely rare and can rather be attributed to wool fat alcohols or to antioxidants like butylated hydroxytoluene (BHT) for instance which are added after the purification process.
Plant or animal sterols
Plant sterols (phytosterols) are structurally related to cholesterol and can therefore replace the animal cholesterol in skin care creams. This explains the excellent skin care characteristics of avocado oil which is rich in phytosterols. The biosynthesis of cholesterol in our body starts with activated acetic acid (acetyl-CoA) via the terpenes geraniol (monoterpene), farnesol (sesquiterpene) and squalene (triterpene). Squalene is a significant re-fattening ingredient of the human sebum and metabolized into lanosterol. Lanosterol, a precursor of cholesterol is also contained in wool fat and has similar emulsifying properties in creams.
Cholesterol is a main component for the human metabolism. It is transported in the blood stream with the help of lipoproteins whose main components are proteins and phosphatidylcholine. Chylomicrons which can be imagined as minuscule emulsion-like droplets help to transport the cholesterol assimilated with the daily nutrition from the small intestines via the lymphatic system into the blood vessels. A significant product of the cholesterol metabolism is pregnenolone, a gestagen which is the base substance for bile acids and steroid hormones.
Bile acids
Bile acids are produced in the liver. They are components of the bile and help break down the food fats. Besides the predominant cholic acid in terms of quantity, there are also desoxycholic acid and several other bile acids.
They are linked with amide bonds with the amino acids taurin and glycin, which, in case of cholic acid for example, results in taurocholic and glycocholic acid. With their emulsifying effects they facilitate the breakdown process of fat-splitting enzymes. Recently, bile acids have increasingly been used as physiological components in cosmetic products. They are used as anionic emulsifiers for lipid substances and can also support lamellar structures. In the nineties, a combination of bile acids and phosphatidylcholine was described in connection with the production of liposomes. The purpose of these liposomal products among others was to prevent allergic reactions and to treat the dry eye symptom.
Steroid hormones
Progesterone which forms from pregnenolone is the base substance for androgens like testosterone but also for the estrogens estrone and estradiol. Changes in the hormonal balance specifically during puberty, the menstrual cycle and menopause have visible effects on the skin appearance.1,2) In contrast to androgens, estrogens have an aromatic ring. This leads to the fact that the hydroxy group located right at the ring has phenolic characteristics. This specific feature is the reason for its structural resemblance to plant isoflavones (polyphenols) which are also called phytohormones. They show a low estrogen effect. Soybean and red clover based phytohormones are mainly used in antiaging products and skin care products for the blemished skin.3) Contrary to phytohormones, steroid hormones and extracts containing steroid hormones are definitely banned in the EU countries.
Glucocorticoids
The biosynthesis of cortisol (hydrocortisone):
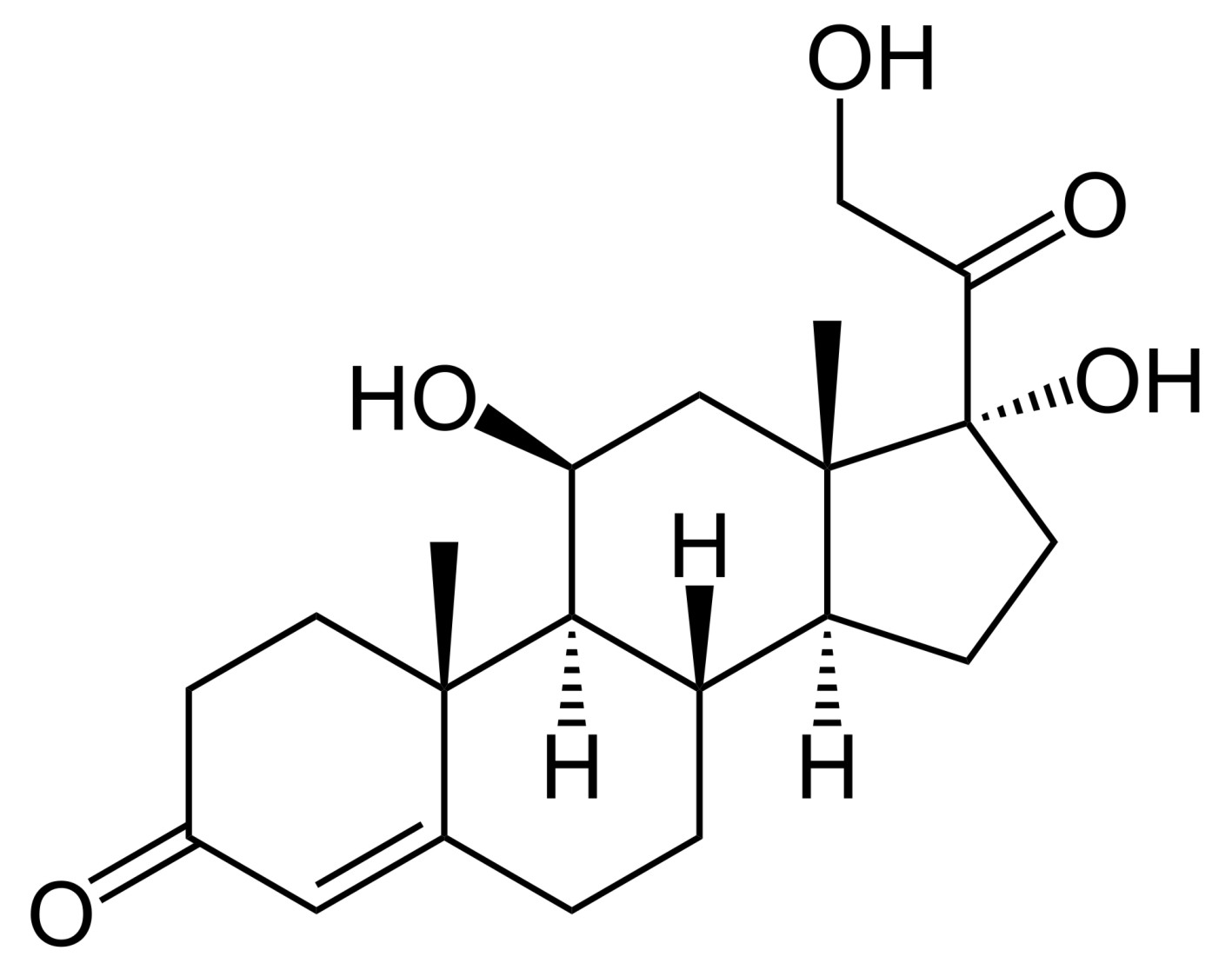
and cortisone from progesterone occurs in the adrenal cortex. Cortisone as such is inactive, cortisol, however, has manifold physiological effects. Orally taken inactive cortisone is transformed in the liver into active cortisol. Cortisol is characterized by its excellent anti-inflammatory and immune-suppressive effects and that is the reason why it is applied in ointments against all kinds of allergies and skin reactions. The skin condition frequently improves within a few days already. A disadvantage though is the atrophic skin condition developing after a long term use. The skin becomes thinner and more permeable for externally affecting irritants and allergens. All in all, the skin becomes more sensible to relapses. In order to reduce these and other side effects, a whole series of artificial corticoids has been developed in addition to hydrocortisone. A further objective is to improve its selectivity for particular indications. This also applies for skin care products. The focus of attention here is the quick breakdown of active agents. On the one hand it is intended to achieve optimal results in the affected skin areas while, on the other hand, systemic effects should be avoided. Said effects may occur if the substances are transported (not intended) through the epidermis. In spite of all the improvements in the field of pharmaceutical drugs it is still recommended to reduce the corticoid dosage as soon as possible after the acute symptoms have disappeared. At the same time it is necessary to provide an accompanying barrier strengthening skin care in order to effectively impede the penetration of allergens and microorganisms.
The anti-inflammatory effect of corticoids can be ascribed to the inhibited phospholipase A2 among others, an enzyme which releases arachidonic acid with its inflammation triggering hormonal metabolites from the natural phosphatidylcholine of the body by hydrolysis.4)
Saponins
Another source for the technical manufacturing of cortisol besides the phytosterol sitosterol is the herbal diosgenin. Diosgenin belongs to the group of herbal saponins with a steroidal ring system. It is also base substance for the industrially produced progesterone.
The term saponin is derived from the Latin word sapo for soap. Like bile acids, saponins also are surface active and have formerly been used for cleansing purposes. In India and other Asian regions the fruits of the wash nut tree (soap nut) with their specifically high saponin content are used still today. Unlike the anionic emulsifying bile acids, the cleansing effect of saponins results from the (glycosidic) linkage of watersoluble sugar residues with the steroidal ring system. That is why saponins can be compared with non-ionic emulsifiers like modern-day sugar tensides which are used for facial cleansing.5)
Besides diosgenin, also ruscin, ruscogenin and neo-ruscogenin are contained in herb extracts as e.g. butcher's broom. The latter mentioned has an astringent and skin-tightening effect and is applied above all for the care of the eye area. The base substance for licorice, glycyrrhizin, also belongs to the saponins and is used in cosmetic applications for skin whitening purposes in case of hyper pigmentation. It is gained from the licorice root (glycyrrhiza glabra).
Cardiac glycosides have a similar glycosidic steroidal structure as saponins. The main active agent digitoxin is extracted from the leaves of the purple foxglove (Digitalis purpurea).
Also related to saponins are the steroidal alkaloids of the solanum family. The most famous representative here is solanine which occurs in potatoes and has a low toxic effect.
Vitamin D3 (cholecalciferol)
In connection with steroids, vitamin D3 is worth mentioning as it is formed from 7-dehydrocholesterol which is a prestage of cholesterol. 7-Dehydrocholesterol occurs in the stratum spinosum and stratum basale of the skin and is transformed into vitamin D3 by influence of UVB light. During this process one of the 4 steroidal rings is opened. The vitamin is also assimilated with the daily nutrition. This is all the more important the less the skin is exposed to sun light and the more sun screens are used. A major source for the vitamin is the consumption of fish especially of those with high fat content like herring, salmon and mackerel.
Dr. Hans Lautenschläger
Further information:
- Kosmetik International 2009 (10), 20-23
- Kosmetik International Best Ager 2009, 26-28
- Kosmetische Praxis 2006 (1), 13-15
- Beauty Forum 2009 (12), 40-47
- Kosmetische Praxis 2009 (4), 12-15
| 
