Structure and metabolism of the epidermis have two major functions: to protect the skin from external impacts and to maintain its hydration and the osmotic balance of the internal tissues. The barrier function of the skin substantially depends on the structural organization of the extracellular lipid matrix of the stratum corneum. Barrier disorders may result in skin diseases such as atopic dermatitis, psoriasis, dry and sensitive skin but also premature skin aging. In order to influence the skin metabolism the skin care preparations need to permeate the skin barrier. One of the approaches in the development of dermatological cosmetic preparations consists of manipulating the lipid layers through penetration modulators in order to improve the permeability of the barrier layers for hydrophilic substances. After the active agents have reached the deeper skin layers, the protective function of the barrier needs to be restored with the help of adequate topical preparations.
Stratum corneum
The function of the skin barrier is determined by the molecular architecture of the lipid layers in the extracellular space. In chemical respect, the barrier consists of a combination of ceramides, long-chained, saturated fatty acids and cholesterol in a molecular ratio of about 1:1:1 [1]. Cholesteryl sulfate and oleate may occur in minor concentrations. These lipids originate from intracellular organelles of the stratum granulosum, the lamellar bodies. The ceramides are formed from sphingolipids (glycosyl ceramides) in an enzymatic process. An adequate hydration is of major significance for a well-balanced lipid metabolism, and an acidic pH-value is significant for the enzyme activity. A particular role in the barrier function is attributed to the ceramides that only occur in high concentration in the stratum corneum. The molecular configuration of the lipids has been illustrated in different models which however failed to explain all the various functions of the skin barrier. A popular didactic model shows the barrier layer as a brick wall with the corneocytes as bricks and the lipid layers as cement [2].
Electron microscopy studies could prove that the lipids form lamellar bilayers in a "wide-narrow-wide"-sequence with the fluid phase (narrow) surrounded by orderly arranged lipid structures [3-9]. According to this particular model, the permeation of substances through the stratum corneum is supposed to occur in the fluid phase. Based on this data, theoretical models on the configuration of the extracellular lipid matrix have been developed. The Bouwstra model shows the narrow band as interconnected long linoleic acid chains of ceramide 1 (ceramide [EOS]), folded short-chained ceramides and cholesterol, whereas both the wide bands are formed by folded long-chained ceramides and cholesterol. The long fatty acid chains of ceramide 1 span through several lipid layers, hence they are the essential structuring component. In this context, both the double bonds in the acyl side chain of ceramide 1 seem to be of particular significance. The substitution of linoleic acid by oleic acid causes considerable disorders in the structure and the barrier function of the stratum corneum.

Fig. 1:
2.260 x magnification of the skin barrier.
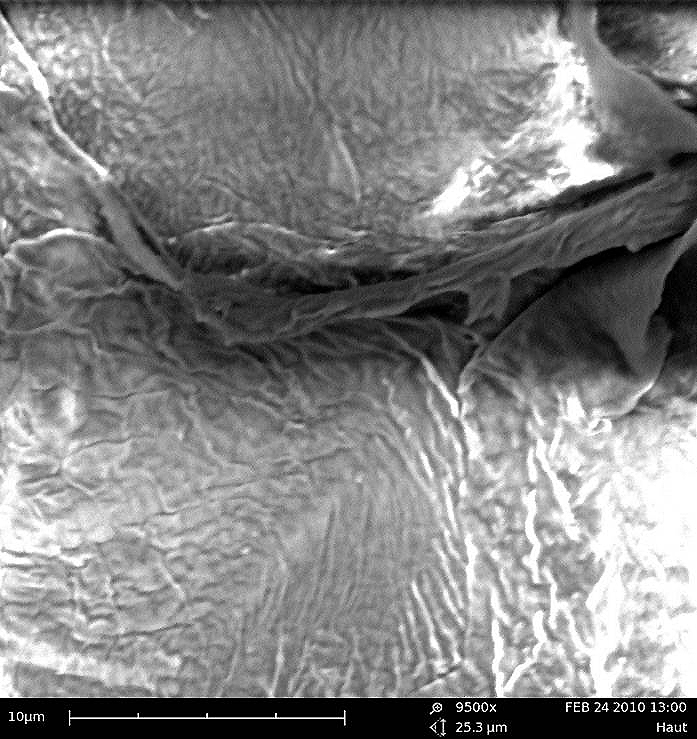
Fig. 2:
9.500 x magnification of the lipid bilayer of the skin barrier.
The innovative Norlén model of the stratum corneum
Norlén has studied the molecular configuration of the stratum corneum lipids employing the innovative cryo electron microscopy. This specific technology utilizes the different electron densities in biological material and thus induced interference effects for the display of membrane structures. It could be shown that the human skin barrier is characterized by asymmetric lipid bilayers. In comparison with model membranes, a bilayer structure has been suggested that consists of stretched ceramides complexed with free fatty acids on the amide bound acyl chains, and of cholesterols on the sphingosine sequence of the ceramides [10].
Due to energetic reasons, this lipid structure of the skin barrier forms a lamellar bilayer. The configuration of lipids originates from biochemical processes in aqueous conditions. Lipids are secreted from lamellar bodies in the extracellular space and through deglycosylation and hydration of glycosyl ceramides, the lipid matrix of the skin barrier is formed [11-13]. Through hydration, the conformation of ceramides modifies from a folded into the stretched form. The elimination of the sugar residue supports this structural modification. While glycosyl ceramides which bind the 5-10 water molecules per lipid molecule only slowly modify their conformation, the flip-flop-movement of ceramides that only bind 0-1 water molecule is considerably faster [14].
Due to this specific configuration of the lipids, an optimally packed bilayer structure is formed. The physiological consequence is a lipid matrix that is nearly impermeable for water. In addition, this protective layer is insensitive to potential dehydration and excess hydration as there is no exchangeable water between the lipid layers.
The structure of the lipid matrix allows the corneocytes to shift within the barrier layer which explains the elasticity of the stratum corneum. Between the different lipid layers there only are hydrocarbon chains that allow a horizontal shifting of the layers against each other which contributes to its elasticity. Due to this unique structure, the thin flexible membrane is rather resilient and impermeable. This new barrier model explains the insensitivity of the skin to hydration and dehydration, to environmental impacts, compression and shear forces.
The recent insights into the ultrastructure of the skin barrier allow for innovative treatment strategies for skin diseases and a more need-oriented development of preparations for the skin protection and the repair of barrier disorders but also of transdermal systems for the active agent transport through the skin [10]. Three different functions of the barrier/active agent system have to be considered in dermatology and cosmetology:
-
Active agents that are effective on the skin surface only as e.g. disinfectants, insect repellents but also decorative cosmetics (epidermal formulations)
-
Topical formulations developed in a way that they penetrate the skin and take effect in the deeper layers of the skin (endodermal or diadermal formulations)
-
Medical drugs and active agents with systemic effects which means that they enter the organism (transdermal formulations).
The target location of a formulation depends on the condition of the barrier layer, on the active agent characteristics (hydrophilic, lipophilic, molecular mass, charge, protein bond) and on the galenics (vehicle function of liposomes and nanoparticles).
![Fig. 3: The innovative Norlén model of the lipid bilayer of the stratum corneum [10]. Fig. 3: The innovative Norlén model of the lipid bilayer of the stratum corneum [10].](https://dermaviduals.de/cms/upload/bilder/Publikationen/Norlen-Modell-2.jpg)
Fig. 3:
The innovative Norlén model of the lipid bilayer of the stratum corneum [10].
Corneotherapy
The corneotherapy is an innovative concept to modulate the barrier function in order to allow an individual treatment of skin problems and to provide effective care for a healthy skin. The term corneotherapy was originally coined by Professor Albert Kligman [15]. Corneotherapy aims at restoring the stratum corneum and improves the function of the skin barrier and the homeostasis of the entire skin. In the case of barrier disorders, harmful substances and microorganisms can reach the deeper layers of the skin where they can trigger inflammations and immunological reactions. In addition, the transepidermal water loss (TEWL) will increase with the result that the skin will dehydrate. Kligman designated the outside-in therapy with the repair and recovery of the stratum corneum through appropriate topical formulations as the primary objective for a dermatological treatment. Subsequently, inflammatory processes in the deeper skin layers are treated with adequate active agents. Contrary to this concept, he depicts the conventional method of applying topical pharmacological active agents in order to treat inflammations in the deeper skin layers without influencing the barrier as inside-out-therapy. Based on the fact that barrier disorders are neglected in this case, relapses will develop. Clinical studies on corneotherapy proved that besides barrier disorders such as atopic dermatitis, also cornification disorders and dermatoses respond to this specific therapy.
Dermato-cosmetic preparations for corneotherapeutic applications
Corneotherapy uses preparations with membrane structures that are chemically and physically adapted to the condition in the stratum corneum. In this context, the presence of phosphatidylcholine seems to be a major prerequisite. The substance plays a decisive role as a natural component of cell membranes. In addition, in its native form it supplies linoleic acid for the formation of ceramide-1. The fluidity of the skin barrier can temporarily be intensified by phosphatidylcholine on the one hand. This specific feature is comparable to the penetration enhancement process achieved by liposomally encapsulated active agents. On the other hand, the fluidity of the barrier layers can be reduced by hydrogenated phosphatidylcholine. Hence, phosphatidylcholine can be utilized to either adjust the skin barrier to fluidity or impermeability, a very significant feature for the practical transport of active agents on the one side and for a natural skin protection on the other side. In its hydrogenated form, phosphatidylcholine is used in membrane creams whereas its native form is utilized in liposomes and nanoparticles [16].
Phosphatidylcholine
The question arises now how these specific features of phosphatidylcholine can be categorized into the new skin barrier model mentioned above. If the unsaturated fatty acid chains of native phosphatidylcholine are hydrogenated, a molecule forms that is comparable to folded ceramides. It is assumed that in the case of barrier disorders, hydrogenated phosphatidylcholine is integrated instead of the missing ceramides and hence repairs and reinforces the barrier. For steric reasons, a very dense packing of the lipid layer consisting of hydrogenated phosphatidylcholine, cholesterol and long-chained saturated fatty acids is achieved. Through hydrogenation the phase transition temperature of native phosphatidylcholine is raised from below 0°C to skin temperature. The ordered gel phase (Pß) widens and the packing density is increased. The barrier is stabilized and barrier damages are repaired.
As opposed to this, the native phosphatidylcholine molecule features kinks in the unsaturated fatty acid residues which increase the volume of the molecule. Consequently, only low packing density is achievable which causes an increased fluidity and a more permeable membrane. The phase transition temperature of native phosphatidylcholine is below 0°C. The phase below the main transition temperature is called an ordered, crystalline phase or gel phase (Pß) while the state above Tm is described as fluid, liquid crystalline or disordered phase (Lα). This main transition, also called endothermic phase transition, leads to a structural modification of the lipid molecules, the so-called "fusion" of chains. The Tm value is determined by the head group, the length and the degree of saturation of the hydrocarbon chains [17]. While the ordered phase Pß is densely packed (all-trans-conformation of the hydrocarbon chains), the liquid crystalline phase Lα corresponds to an irregular packing (gauche-conformation) [18]. The following characteristics can be observed during the phase transition of an ordered gel to the fluid phase [19]:
- Surface extension
- Shortening of hydrocarbon chains
- Extension of the lipid bilayer
- Rapid lateral diffusion
- Reduction of Van der Waals interactions
With the increase of either chain length or saturation, also the phase transition temperature (Tm) rises. At body temperature, the native phosphatidylcholine occurs in an amorphous liquid-crystalline phase. The barrier layer is loosened and hence becomes more permeable for liposomally encapsulated active agents. The physical characteristics of native phosphatidylcholine facilitate the formation of spherical structures such as liposomes or nanodispersions while hydrogenated phosphatidylcholine rather forms lamellar structures due to thermodynamic reasons. Or, in other words, membrane creams (hydrogenated PC) stabilize and repair the barrier while liposomes and nanodispersions (native PC) loosen the barrier and make it more permeable.
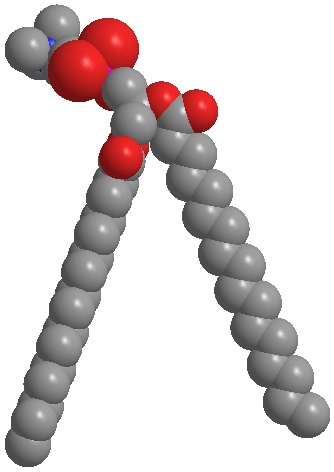
Fig. 4a:
Hydrogenated phosphatidylcholine forms lamellar structures similar to the skin barrier.
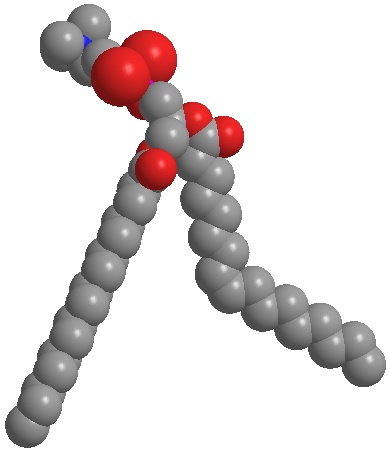
Fig. 4b:
Native phosphatidylcholine (unsaturated) forms spherical structures such as liposomes and nanodispersions. Phosphatidylcholine can be used to adjust the skin barrier to penetrability or impermeability.
Cholesterol
Due to the amphiphilic character and the rigid condensed basic structure, cholesterol shows different features such as stabilization and fluidization of lipid bilayers [20-23]. In cholesterol-containing phospholipid bilayers the viscosity is reduced. The rise of fluidity involves an increased surface tension. Since this structure is energy inefficient because of the increased surface tension, cholesterol-containing phospholipid layers rather form lamellar membrane structures than spherical liposomes.
Free fatty acids
Free fatty acids are of major significance for the integrity of the lipid bilayers and for the normal barrier function. According to the studies of Norlén, free fatty acids attach to the amide-bound acyl chains of the stretched ceramides and thus form a functional unity with cholesterol.
These unique features of the skin barrier resulted in the development of skin care preparations for the treatment and prevention of skin damages. Based on the corneotherapy concept established by Professor Kligman, phosphatidylcholine-containing membrane creams and liposomally and nanoparticularly encapsulated active agents have been developed in order to specifically influence the skin barrier and adjust it to a permeable or non-permeable state according to the required treatment concept.
References
-
Wertz P et al. "Confidence Intervals" for the "true" lipid compositions of the human skin barrier? In: Forslind B, Lindberg M (eds) Skin, Hair, and Nails Structure and Function (2003). Marcel Dekker, New York, 85-106.
-
Michaels AS et al. Drug permeation through human skin. Theory and in vitro experimental measurements. AICH J.(1975) 21 (5), 985-996.
-
Breathnach AS. Aspects of epidermal structure. J. Invest. Dermatol. (1975) 65, 2-12.
-
Breathnach AS et al. Freeze fracture replication of cells of stratum corneum of human cells. J. Anat. (1973) 114, 65-81.
-
Madison KC et al. Presence of intact intercellular lamellae in the upper layers of the stratum corneum. J. Invest. Dermatol. (1987) 88, 714-718.
-
White SH et al. Structure of lamellar lipid domains and corneocyte envelopes of murine stratum corneum. An x-ray diffraction study. Biochemistry (1988) 27, 3725-32.
-
Bouwstra JA et al. Structural investigations of human stratum corneum by small-angle X-ray scattering. J. Invest. Dermatol. (1991) 97, 1005-1012.
-
Bouwstra JA et al. Structure of human stratum corneum as a function of temperature and hydration: a wide-angle x-ray diffraction study. Inter J Pharmaceut (1992) 84, 205-207.
-
Bouwstra JA. Phase behavior of stratum corneum lipid mixtures based on human ceramides: The role of natural and synthetic ceramide 1. J Invest Dermatol (2002) 118(4), 606-617.
-
Iwai I et al. The human skin barrier is organized as stacked bilayers of fully extended ceramides with cholesterol molecules associated with the ceramide sphingoid moiety. J Invest Dermatol (2012), doi: 10.1038/jid. 2012.43, 1-11.
-
Caspers PJ et al. In vivo confocal raman microspectroscopy of the skin: noninvasive determination of molecular concentration profiles. J Invest Dermatol (2001) 116, 434-442.
-
Norlén L. Skin barrier formation: the membrane folding model. J Invest Dermatol (2001) 117, 823-829.
-
Al-Amoudi A et al. Nanostructure of the epidermal extracellular space as observed by cryo-electron microscopy of vitreous sections of human skin. J Invest Dermatol (2005) 124, 764-777.
-
Steck TL et al. Probing red cell membrane cholesterol movement with cyclodextrine. Biophys J (2002) 83, 2118-2125.
-
Lautenschläger H. Korneotherapie - Bindeglied zwischen Dermatologie und Kosmetologie. KOKO Kosmetikvertrieb GmbH & Co. KG, Leichlingen (2011).
-
Lautenschläger H. Geschichte und aktuelle Gesichtspunkte der Korneotherapie. Kosmetische Medizin (2005) 26 (2), 58-60.
-
Phillips MC et al. Monolayer characteristics of satured 1,2-diacyl phosphatidylcholines (lecithins) and phosphatidylethanolamines at the air/water interface. Biochim Biophys Acta (1968) 163, 301-313.
-
Winter R. Struktur und Dynamik von Modell-Biomembranen. Chemie in unserer Zeit (1990) 24, 71-81.
-
Keough KMW et al. Gel to liquid-crystalline phase transitions in water dispersions of satured mixed-acid phosphatidylcholines. Biochem (1979) 18, 1453-1461.
-
Vist M et al. Phase equilibria of cholesterol/ dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry (1990) 29, 451-464.
-
Smaby J et al. Phosphatidylcholine acyl unsaturation modulates the decrease in interfacial elasticity induced by cholesterol. Biophys J. (1997) 73, 1492-1505.
-
Worthman LA et al. Cholesterol in condensed and fluid phosphatidycholine monolayers studied by epifluorescence microscopy. Biophys J. (1997) 72, 2569-2580.
-
Tanaka K et al. Lipid lateral diffusion in Dilauroylphosphatidylcholine/Cholesterol mixed monolayers at the air/water interface. Langmuir (1999) 15, 600-606.
Figures
-
Fig. 1: 2.260 x magnification of the skin barrier.
-
Fig. 2: 9.500 x magnification of the lipid bilayer of the skin barrier.
-
Fig. 3: The innovative Norlén model of the lipid bilayer of the stratum corneum [10].
-
Fig. 4a: Hydrogenated phosphatidylcholine forms lamellar structures similar to the skin barrier.
-
Fig. 4b: Native phosphatidylcholine (unsaturated) forms spherical structures such as liposomes and nanodispersions. Phosphatidylcholine can be used to adjust the skin barrier to penetrability or impermeability.
Dr. Hans-Ulrich Jabs | 
